Lower Costs, Faster Enrollment, Happier Sponsors
Use Ryght's AI Digital Twin platform to secure your competitive edge, bring life-changing therapies to patients sooner, and achieve unparalleled cost efficiencies.
Reduce operational costs & resources
Ryght AI's platform allows your existing team to manage larger portfolios while increasing value, improving your operational efficiency and profit margins.
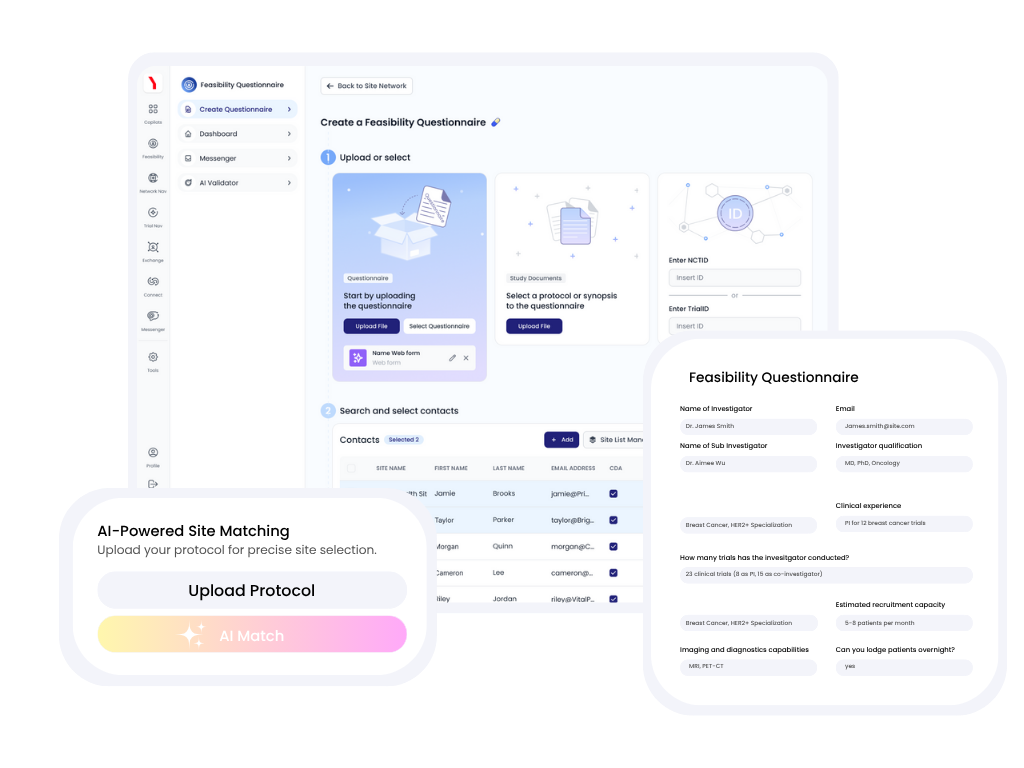
Cut months of manual research down to minutes. Our AI-powered platform instantly analyzes the protocol and scans 100,000 AI Site Twins to identify optimal sites, eliminating the need for expensive data subscriptions and costly consultants.
Feasibility questionnaires are pre-populated with data from each sites' corresponding AI Site Twin. Sites only need to complete a quick review and confirm responses. This reduces traditional 4-6 week feasibility cycles to just days, freeing up your team for higher-value activities.
Advanced AI matching eliminates costly site selection mistakes that lead to poor enrollment, protocol deviations, and study delays. Our precision matching reduces the risk of selecting underperforming sites that drain resources throughout the study lifecycle.
Our comprehensive AI Site Twins provide deep insights into site capabilities before you invest in costly visits and extensive due diligence processes, allowing you to focus resources only on the most promising candidates.
Deliver exceptional value to your clients
Demonstrate clear ROI to your clients through faster timelines, reduced costs, and improved study success rates that keep customers coming back.
Compress traditional 6-12 month site identification and selection timelines into weeks. Your sponsors can advance their programs to market faster, translating saved time into millions of dollars in extended market exclusivity.
Our AI matching ensures optimal alignment between protocol requirements and site capabilities, leading to higher enrollment rates, better protocol compliance, and reduced study risk for your biopharma clients.
Gaining access to AI Site Twins of 100,000 clinical research sites across the globe means you can confidently take on international studies and help clients expand into new markets without the traditional barriers of local knowledge and network limitations.
Explore the Ryght platform
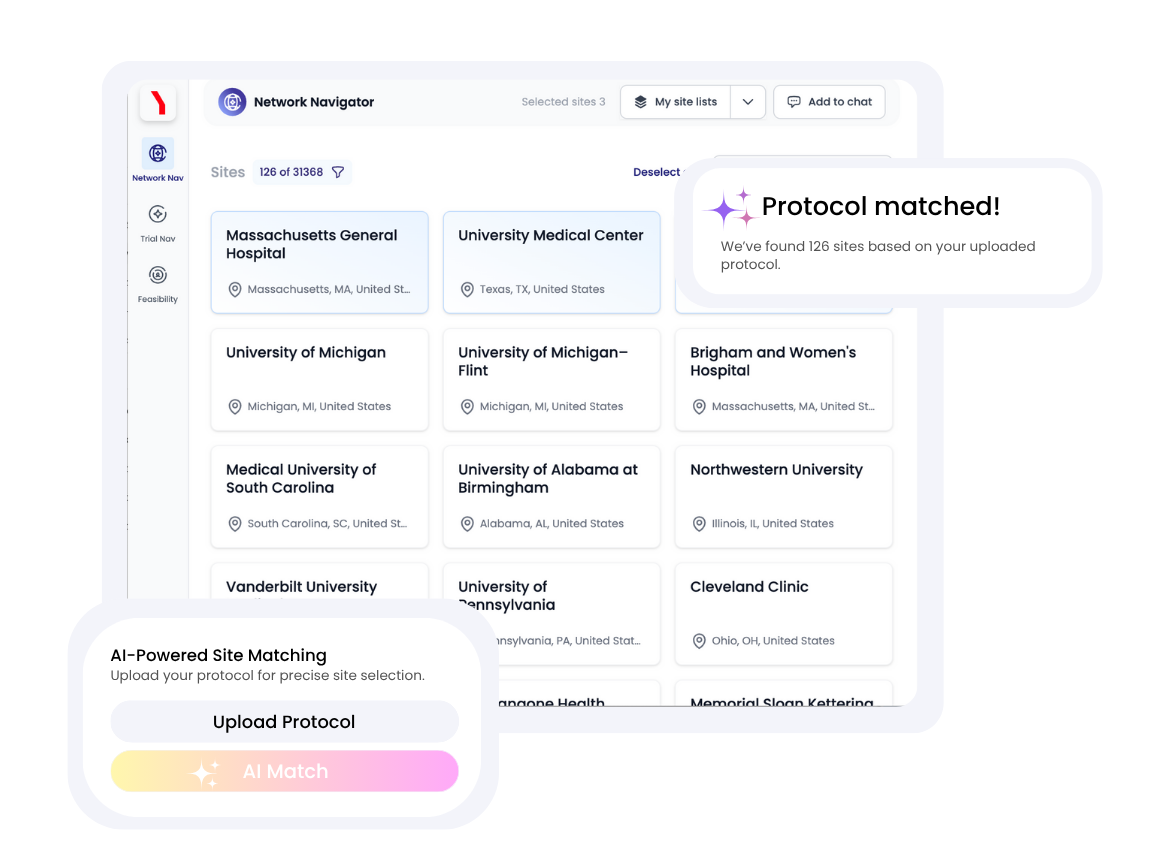
Leverage AI-powered digital twins of every clinical research site in the world to instantly find the locations that best match your protocol’s needs.
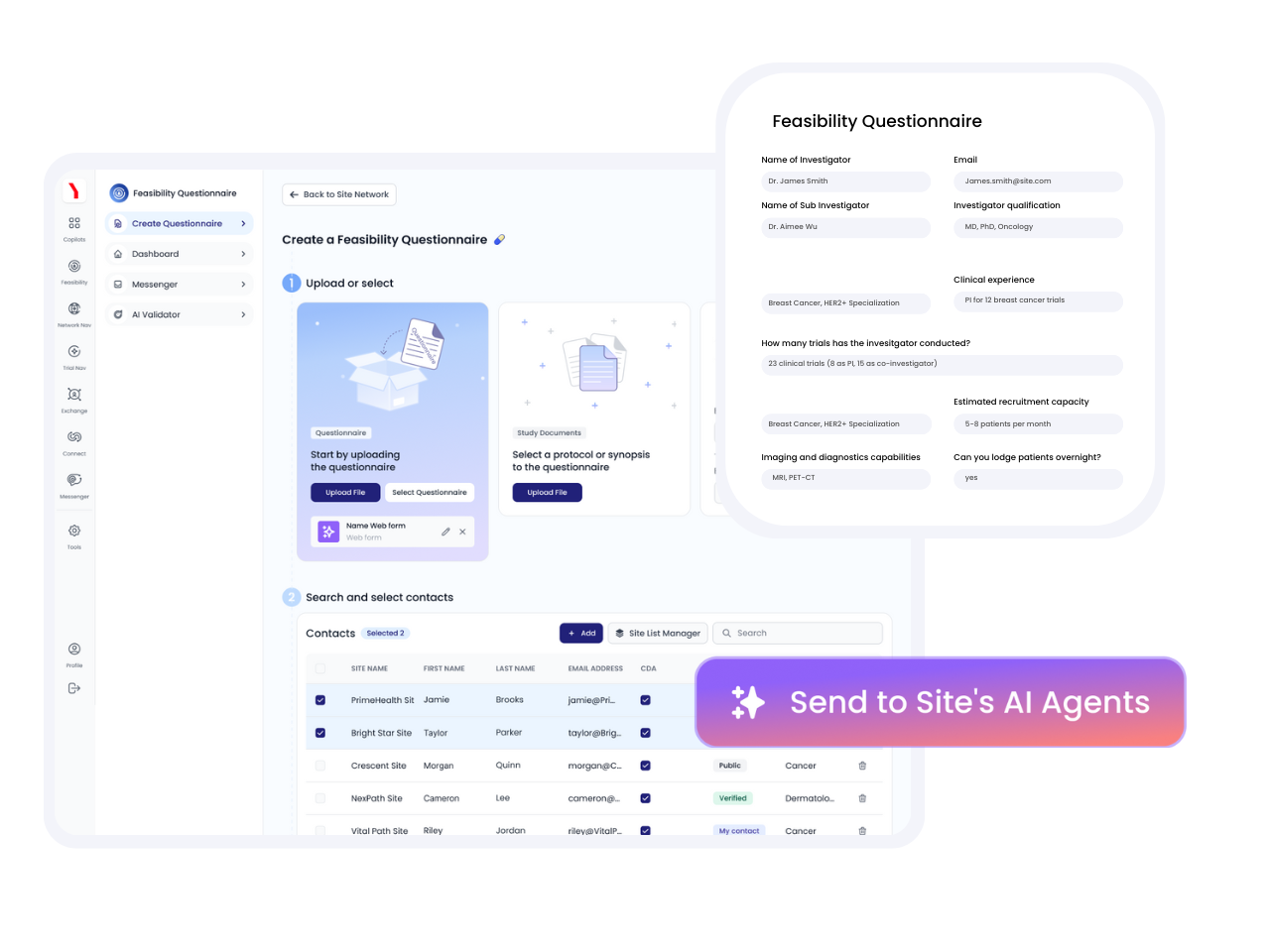
Automatically send qualified sites pre-populated feasibility questionnaires powered by data from our AI Site Twin network. Track and compare all responses in real-time.
.png)
Ryght will design, build, and deploy industry-specific agents tailored to your exact requirements, from turnkey solutions based on proven use cases to fully custom builds that solve your unique challenges.
Available on the Microsoft Azure Marketplace
Ryght is available on Microsoft Azure Marketplace, making it easy for sponsors and CROs to use existing cloud commitments, simplify billing, and increase cost efficiency.

Test Ryght on an old protocol to see how we stack up.
Related resources
Ryght AI Announces Partnership with Biorasi to Transform Feasibility Accuracy in Clinical Trials
Ryght AI, an AI clinical trial developer, today announced a partnership with Biorasi, a global clinical research organization (CRO) specializing in dermatology, oncology, neurology, and nephrology studies...
How will CROs benefit from Generative AI?
Stay connected
Subscribe to be the first to know about product updates, news and announcements.
